Software as a Medical Device
As an ISO 13485 certified medical software company, we deliver high-quality software as a medical device solutions that comply with industry standards. Our robust quality management system (QMS) and proven experience ensure that our applications meet the stringent requirements of CE, FDA, and MDR regulations
Our specialists will design your tailored software solution, adhering to regulatory and medical requirements.
Medical Device development services we provide
Specialized medical software solutions tailored for healthcare enterprises developing digital health innovations aimed at MDR or FDA approval. Moreover, if your organization is creating software that functions as or within a medical device, our expertise can be invaluable. During the project we create documentation in accordance with medical standards which will support and accelerate the certification process of your solution in the future.

ISO 13485 software
The solutions we have developed for our clients have been successfully accepted by regulators and marketed.
Quality Management System
Our internal Quality Management System (QMS) ensures that every step of the process is properly documented and tested.
Medical regulations
We guide you through MDR, IVDR, FDA, or DiGA compliance, from coding to documentation.

Certified medical software development
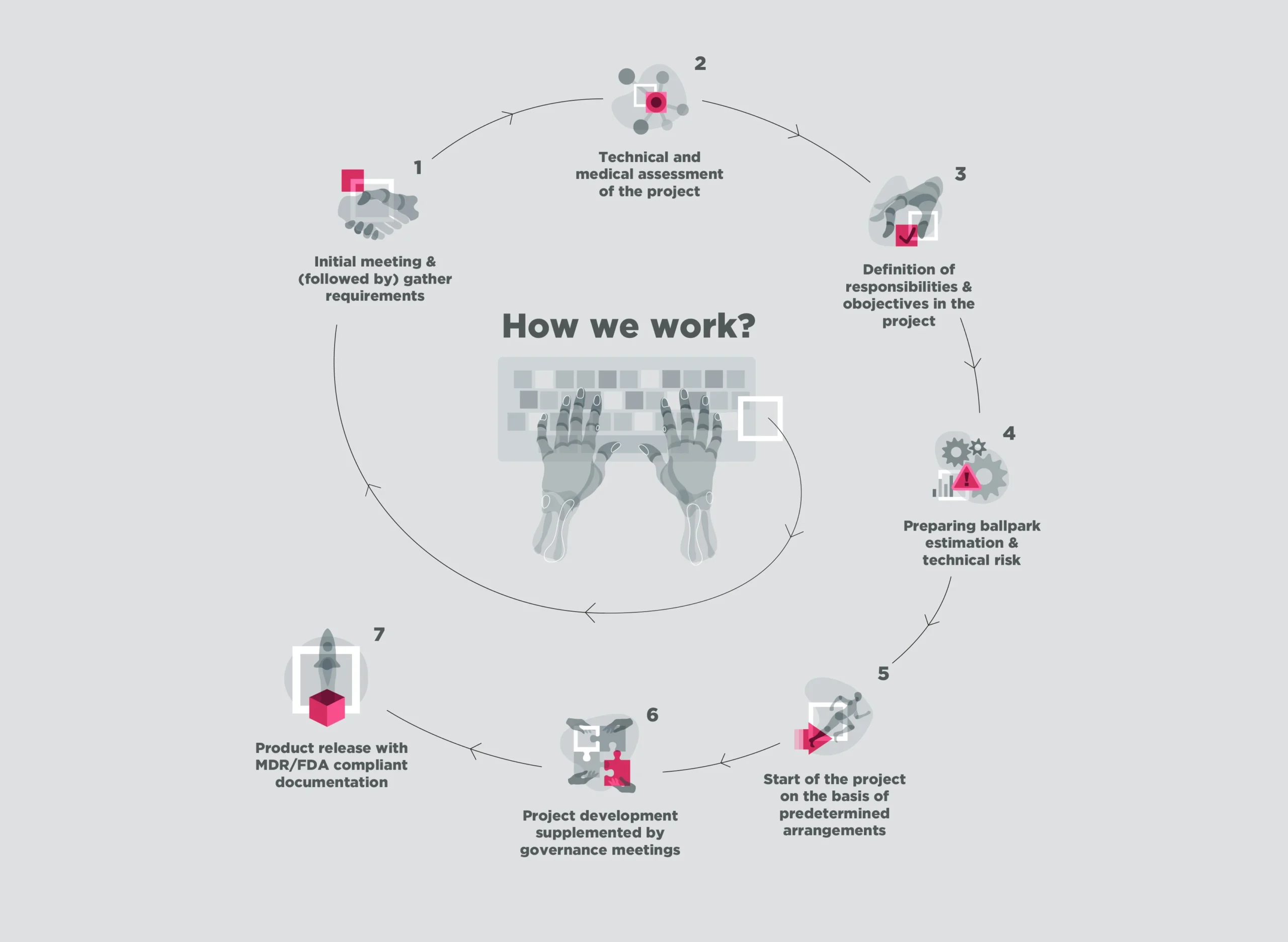
Full-cycle medical application development support, followed by regulatory-compliant technical documentation and risk assessment. We will guide you through the precise definition of objectives and responsibilities, qualification of the medical device class and delivery of MDR and FDA compliant documentation.
Firstly, we assist in compiling comprehensive software documentation. Subsequently, we guide you through the intricacies of UX/UI design. Additionally, we help strategize your technology and architecture plans. Finally, we support the development of your digital product, ensuring compliance with MDR in Europe and the UK or FDA in the USA certification standards throughout the process. In essence, our end-to-end services streamline your journey toward regulatory approval.
Software as a Medical Device solutions
There’s a lot we can help you with.
Custom medical device software development.
We have a solid understanding of what software as a medical device manufacturing is all about. Thanks to our experience, we are able to assist you at every stage of the project and will provide you with all the technical documentation that is required during certification.
Proven competencies in Software as a Medical Device development
Proven competencies in Software as a Medical Device development
Let’s work on your challenges together!

Contact us:

Let’s work on your challenges together!
Contact us: